ARPA Bio
in tune with innovative solutions for early therapeutic intervention...
How do these antibody molecules distinguish from earlier, clinically approved antibodies ?
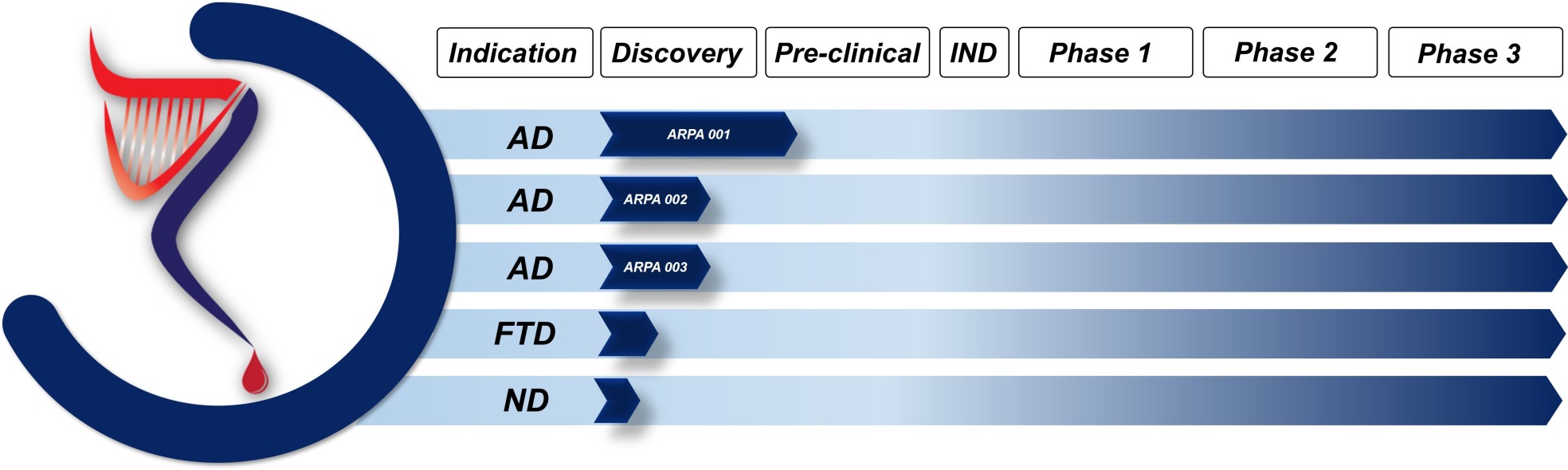
ARPA Bio's product portfolio consists of a novel monoclonal antibody molecule (ARPA001) with unique binding properties against early seeds of neurotoxic amyloid species. ARPA001 binding proptery distinguishes from earlier developed conformation specific or the clinical antibodies, since ARPA001 does not bind large, oligomeric or protofibrillar species but rather small molecular seeds prior to the appearance of oligomers. Our studies have shown, that these specific, early species are not detected by the current, clinical antibodies and therefore creates a loophole for early therapeutic or preventive intervention. Moreover, our lead antibody molecules do not bind non-toxic, amyloid-beta monomers, which is an additional, significant improvement when compared to earlier developed antibodies. Other antibodies (ARPA002 & ARPA003), with highly specific binding properties against different amyloid seeds serve currently as back-up molecules and will be subjected to future preclinical evaluation to maximize the therapeutic efficacy. (AD: Alzheimer, FTD: Frontotemporal Dementia, ND: not disclosed)
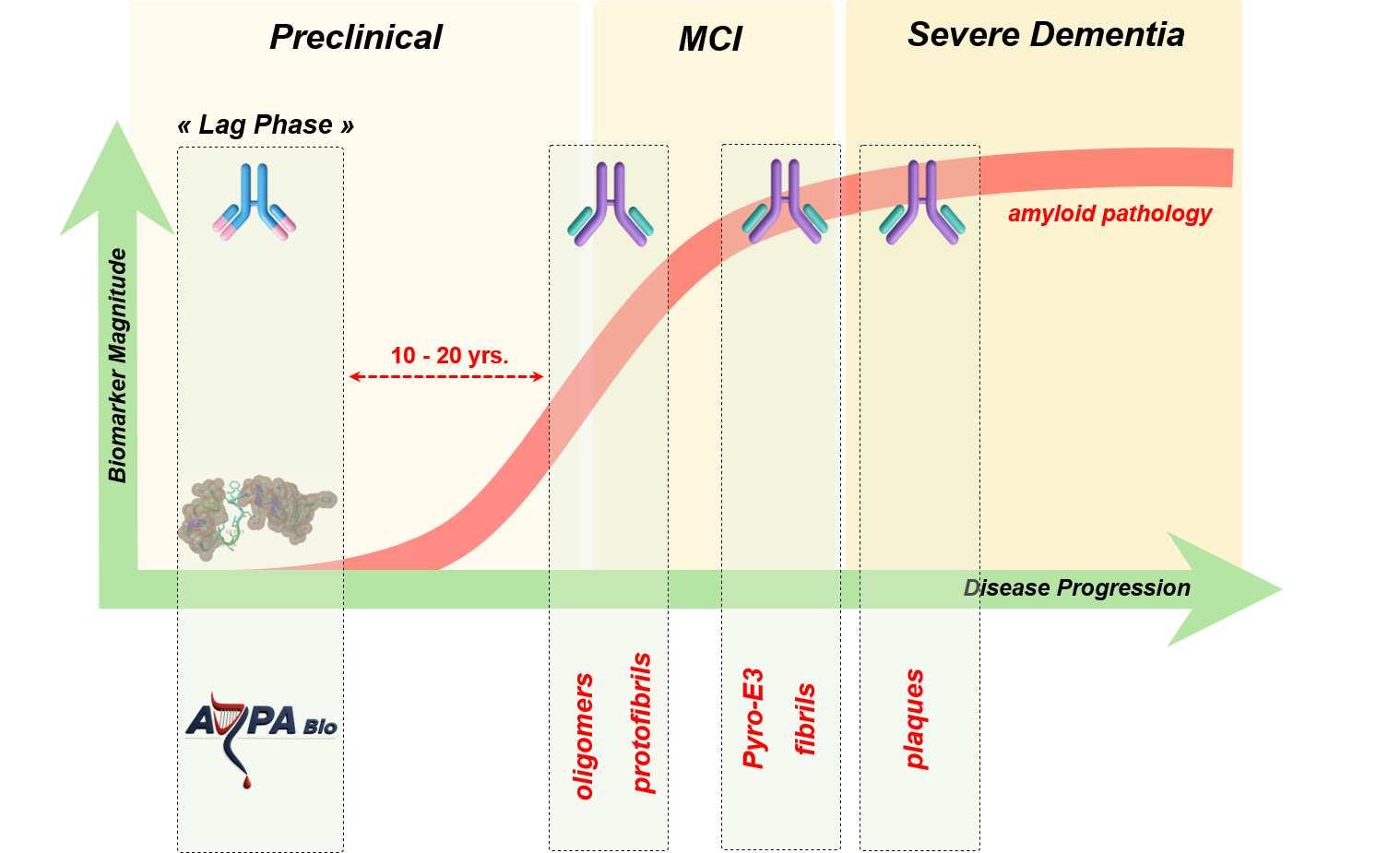
Simplified overview of ARPA Bio's therapeutic strategy using immuno-therapy to target early seeds of amyloid species during the long preclinical phase (lag phase 10-20 years) of the disease. (MCI: mild cognitive impairment)
